The atomic number is the number of protons in the nucleus of an atom therefore it is the same to the charge number of the element. Electron Configuration Li 1 3 1 1s2 2s1 N 5A 7 5 1s2 2s2 2p3 F 7A 9 7 1s2 2s2 2p5 Ne 8A 10 8 1s2 2s2 2p6 Na 1 11 1 1s2 2s2 2p6 3s1 Mg 2 12 2 1s2 2s2 2p6 3s2 Al 3A 13 3 1s2 2s2 2p6 3s2 3p1 Cl 7A 17 7 1s2 2s2 2p6 3s2 3p5 Ar 8A 18 8 1s2 2s2 2p6 3s2 3p6 K 1 19 1 1s2 2s2 2p6 3s2 3p6 4s1 Ca 2 20 2 1s2 2s2 2p6 3s2 3p6 4s2 Br 7A 35 7 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5.

Dublin Schools Lesson Electron Configurations Using The Periodic Table
Up to 24 cash back The atomic number of the elements on the periodic table are organized chronologically starting with Hydrogen with the the atomic number of 1 going from left to right.

. Boron B has an electron configuration 1s²2s²2p¹. Moselys table was arranged this way. Electron Configuration Pattern - 17 images - iron orbital and bonding info chemistry notes on atomic structure electron electron waves.
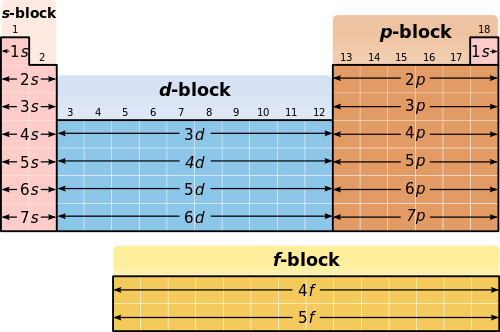
Ns2np6configurationexception-He Transition elements - 1B 3B - 8B d- block Lanthanidesactinides - f-block. Copyright McGraw-Hill 2009 8. This example warns you that there are exceptions to the general pattern of electronic.
Classification of Elements Main group elements - representative elements Group 1A- 7A Noble gases - Group 8A all have. 72 The Modern Periodic Table. The table below for the main group elements is set out just like the Periodic Table of the elements.
Trends in the Number of Valence Electrons. One way to check if the notation is correct for a given element is to see if the sum of the exponents in the notation equals the number of electrons in an atom of that element. The electronic configuration in shell notation is given for an atom of each of the elements.
The main properties that can be compared is the melting. The total electron configuration is thus Nb Kr4d 3 5s 2 Note that the principal quantum number of the d subshell is 4 one less than the number of the period. Electron configurations are the summary of where the electrons are around a nucleus.
Chlorine is found in the group 17 the halogens on the periodic table. The electrons in the valence shell highest energy level are given in red. There are two electrons in the s subshell and six in the p subshell.
The shape of the periodic table mimics the filling of the subshells with electrons. Periodic Patterns in Electron Configurations In each column write the element name and the Electron Dot Diagram for the element that appears in that place on the periodic table see samples below Group 1 Group 2 Group 13 Group 14 Group 15 Group 16 Group 17 Group 18 Period 2 Lithium Be b Carbon n o f n e Period 3 Na Mg AI SI P S CI Argon. The chloride ion Cl on the other hand has an additional electron for a total.
Which sublevels are filling up as one goes. Learn vocabulary terms and more with flashcards games and other study tools. First row 1s 2 Second row 2s 2 2p 6 Third row 3s 2 3p 6 Fourth row 4s 2 3d 10 4p 6 Fifth row 5s 2 4d 10 5p 6.
Period row Group column Use the table on your book cover which shows only. The anomalous electronic configuration of chromium and copper is interpreted as the displacement of 1 electron from an s orbital into a d orbital. We consent this nice of Periodic Table Electron Configuration Pattern graphic could possibly be the most trending subject following we part it in google improvement or facebook.
With He the n 1 shell is filled. The electronic configurations of the elements in group 1. As we learned earlier each neutral atom has a number of.
In this lecture we continue the discussion of Quantum Numbers and their use in Electron Configurations as well as the relationship of electron configuration to the periodic properties of the elements. According to the Aufbau principle the electrons of an atom occupy quantum levels or orbitals starting from the lowest energy level and proceeding to the highest with each orbital holding a maximum of two paired electrons. These two elements have only one electron in the 4 s subshell because the second electron was promoted into a 3 d subshell.
111 rows We now move farther right into the 4d subshell region of the periodic table and count over three spaces Y Zr Nb to reach Nb. One of the many patterns contained in the periodic table is that of electron configuration. The atoms of all group 1 elements have similar chemical properties and reactions because they all have one electron in their outer shell.
With an atomic number of ten neon has two electrons in the main shell and eight electrons in the second shell. Elements arranged by increasing atomic number show repeating pattern of properties. Atomic radii differ in an anticipated and logical way over the periodic table.
Their electron configurations are 1 s 1 and 1 s 2 respectively. The neutral atom chlorine Z17 for instance has 17 electrons. How many electron configurations does a chlorine atom have.
The arrangement or electron configuration forms an obvious pattern on the periodic table on the simply by looking at the periodic table. Start studying Unit 2B The Periodic Table and Electron Configurations. SOLUTION We can do this by simply moving across the periodic table one row at a time and writing the occupancies of the orbital corresponding to each row refer to Figure 629.
Elements with 1 valence level electron H Li Na have an ionic charge of 1 and lose 1 electron. Later you will use these patterns to determine the order in which electrons fill the orbitals of an atom. As you complete the activity keep the following in mind.
The truncated periodic table shown above provides the orbital electronic structure for the first eighteen elements hydrogen through argon. Excepting helium elements with 2 valence level electrons Be and Mg have an ionic charge of 2 and lose 2 electrons. Let us start with H and He.
Therefore its ground state electronic configuration can be written as 1s 2 2s 2 2p 6 3s 2 3p 5. The Modern Periodic Table. They are in column 1 of the periodic table.
In this activity you will identify these patterns. The electron pattern for neon for instance is 1s 2 2s 2 2p 6. Metallic elements in group 2 of the periodic.
The electronic configuration of elements will be 3 2 1 11 2 8 1 19 2 8 8 1 37 2 8 8 18 1 55 2 8 8 18 18 1 The pattern shows that all elements have 1 electron in their outermost valence shell and all these elements belong to alkali metals. Write the electron configuration for the element bismuth atomic number 83. The electron configuration follows a periodic order where lower-level shells are filled in before higher-level shells.
These two elements make up the first row of the periodic table Figure 97. They are in column 2 of the periodic table. Previous page with a little practice you will be able to find the electron configuration of any element Which groups are the representative main group elements.
Chemistry Periodic Table Electron Configurations - 18 images - electron configuration of elements read chemistry google image result for electron configuration chart electrons electron configurations the periodic table.

Electron Configurations The Cavalcade O Chemistry

Electron Configuration And The Modern Periodic Table Examples Pedia

Writing Electron Configurations Using Only The Periodic Table Youtube

Electron Configurations And Magnetic Properties Of Ions Introduction To Chemistry

Electron Configurations In Atomic Energy Levels Video Lesson Transcript Study Com
How To Use The Periodic Table To Write Electron Configurations For Each Element Quora
Electron Configurations And Magnetic Properties Of Ions Introduction To Chemistry

0 comments
Post a Comment